At the occasion of our symposium to celebrate our 10th Anniversary, Tanguy Schmitz, president of BAPIE (Belgian Association of Parallel Importers and Exporters), did a presentation to explain how parallel trade works in Belgium.
“BAPIE was created 10 years ago between two parallel import companies. Among the founders, myself and Gregory Tjoens. BAPIE is located at 26, rue des Deux Eglises in Brussels and shares its offices with Affordable Medicines Europe, the coupole organisation of parallel trade in Europe. BAPIE has developped through the years and gathers today around 10 companies active in parallel import, export or distribution of medications in Belgium.
All BAPIE members are also members of Affordable Medicines Europe, and we follow the “good parallel distribution guidelines” defined by the European Medicine Agency (EMA). It defines the quality requirements of the pharmaceutical wholesalers regarding the supply chain, the storage, the transportation conditions and the temperature. On top of those legal requirements, we impose to our members to pass an independant TÜV audit focussed mainly on the quality of supply. This audit is done every two years. A failure in this audit lead to corrective actions, exclusion, or communication to the autorities.
The patient is central in our mission. Patients must receive the medications they need. We feel, as BAPIE, a particular mission, an enthusiastic mission, to fight against shortages in Belgium. We call that: “fill the gap“.
Affordability is another crucial element of our mission. Affordability means competition, choice between different suppliers. Away from monopole. Away from artificial partition of Europe along to the old countries’ borders. From competition, free circulation in Europe, a better price for the patient and for the social security can emerge. We do not know a better recipe, to have the best possible price, than competition.
BAPIE is structured in three pillars: 1) parallel import, 2) parallel export and 3) parallel distribution.
- The parallel importers (PI) obtain marketing autorisations (licenses) to put medications on the market from FAGG/AFMPS. This is the national route. A dossier is filed at the agency following the rules depicted in the Royal Decree of 19 April 2001. This concerns an identical product to the reference (made by the same company, in the same manufactory) or equivalent product, if there are minor differences in the excipients. The equivalent status is delivered by the official Belgian medication commission.
- The parallel exporters are short-range wholesalers. They have obtained their licence to export from FAGG/AFMPS. They will never export medications which are in shortage in Belgium: Belgian patient first. This is the Public Service Obligation (PSO). This Public Service Obligation is reinforced by the recent “export ban” RD of 18/01/2023 which defines critical medications of which exportation is subject to a pre-notification.
- The parallel distributors (PD) represent European wholesalers. They obtain the licences to market the products they appy for from the EMA. The products for which they obtain European licences are identical to the reference product, it means that they are made in the same manufactory by the reference pharmaceutical company itself.
Concerning the importance of parallel trade in Belgium, officinal pharma sales represented 3,3 billion euros (ex-factory prices) in 2022 in Belgium. Our sales represented only 125 million euros, an extremely limited portion of the 3,3 billion mentioned. This represent less than 4 % of the market. So, parallel sales exist in Belgium, competition exists for some medications, but not for the many of them. Parallel importation is still small. We speak of small numbers. We play the role of “Luis in de pels” (NL), or “Mouche du coche” (FR), or Robin Hoods (EN).
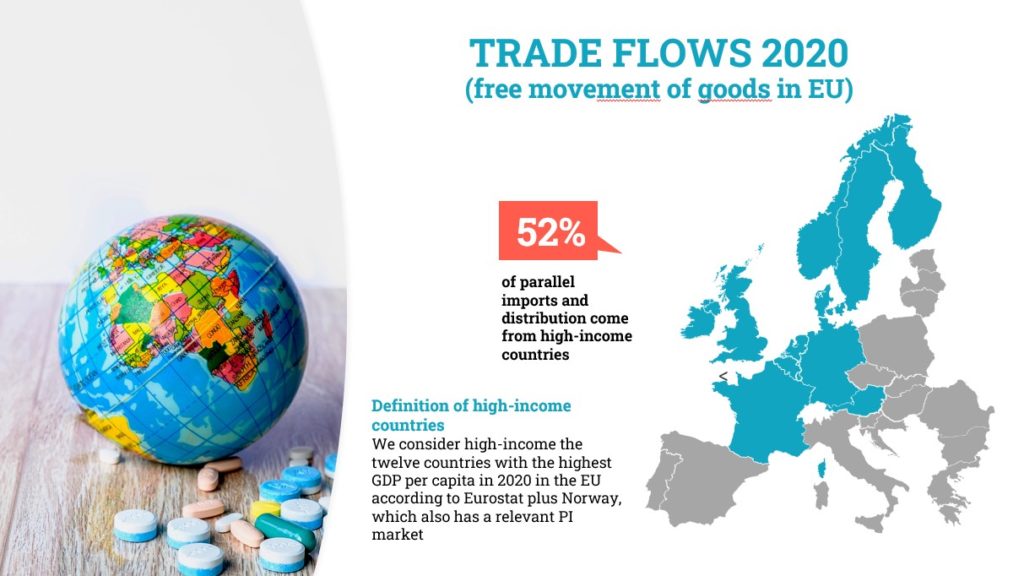
Our European coupole association, Affordable Medicines Europe, studied in 2020 the trade flows of the parallel trade in Europe. First, one should remember that we live in Europe and that a fundamental principle is free trade, free circulation of the goods and medications. The vast majority of this trade occurs among twelve rich, North-European countries. Some products are cheaper in Germany, Sweden, the Netherlands than in Belgium and BAPIE members import them from those countries. We do not import a lot of medications from poorer countries of Europe, such as Greece, Bulgaria or Romania.
If there are national export ban regulations, our members respect them, and they will not create shortages in those countries. Our suppliers respect also the PSO and, in our TÜV audit, we verify that they respect their PSO obligations and that they do not import goods which are targeted by a local export ban rule.
Concerning the shortages, following the Technopolis study, commissioned by the European Commission, in 90-95 % of the occurrences, a shortage is due to something going wrong in the local, national supply route. Rarely, but it happens, a shortage is at European level (default of an API, worldwide crisis…).
What does parallel import? It spots the European countries where stocks are in excess and transport them where there are in shortages. Parallel trade fills the gap.
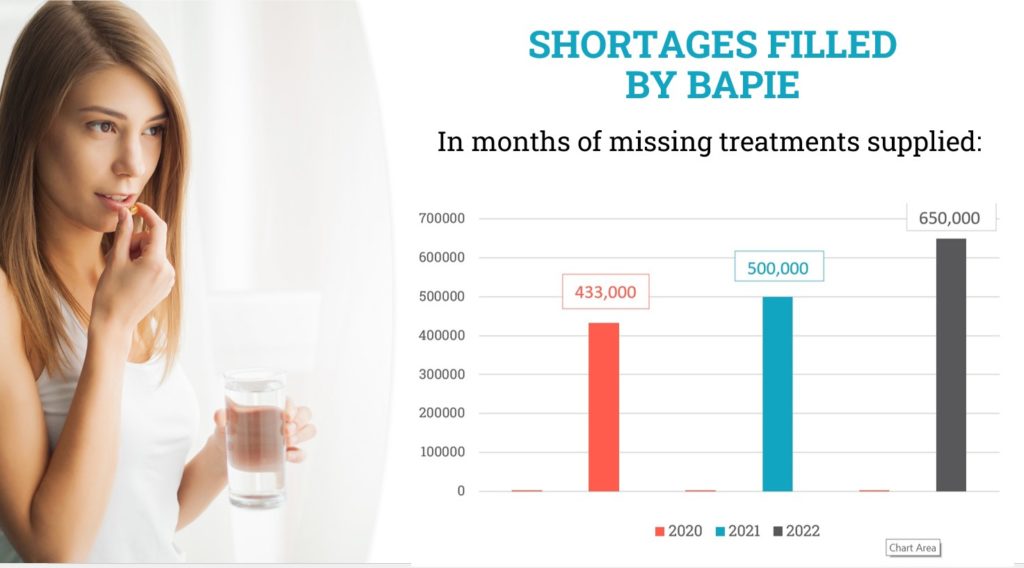
Based on the official AFMPS/FAGG “PharmaStatus” list of products, missing in Belgium, we have calculated the number of boxes of 30 pills we were able to import. We imported 650,000 boxes of those missing products in 2022, it means between 1,000 and 2,000 “30 pills boxes” per day! We see that this number is increasing at the same rythm at which we receive new importation licences. By doing that, we not only relieve the Belgian patients, but also the local pharmacist of the long and difficult job of finding those products abroad for individual patients.
Parallel importers, parallel distributors play a silent, efficacious and permanent search in filling shortages. This is the most natural and effective way to fill them. By offering an alternative supply to the medication in shortage, we help also to compliance: the patient can be kept on his medication, precisely following the doctor’s prescription.
Concerning the causes of shortages, based on the figures given to AFMPS/FAGG by the industry, it appears that around 75 % of the shortages are due to internal difficulties of the industry (delivery, production, logistic). Parallel trade is never mentioned as a source of shortages.
Shortages, but also quotas : there is a discrepancy around the figures of shortages: around 300 missing medications according to FAGG/AFMPS (PharmaStatus) and more than 1200 according to the full-line wholesalers.
Official vs. real? Official meaning lacking during more than 30 days and communicated/verified by the agency. “Real” meaning not delivered despite the orders of the full-line wholesalers. Nine hundred products would be “on quotas”, not delivered directly to the pharmacists and bypassing the full-line wholesalers. The legal obligation to deliver to full-line wholesalers is not respected.
Pharmacists are submerged by additional administrative work and industry is progressively taking over the control of the whole supply chain. To the benefit of the patients? Patients feel unsecure and they start to make provision, like hamsters. We call that “hamsterisation”. This creates overstock with some patients and shortages for others… As a whole, the quota policy creates shortages and obliges the pharmacists to make individual orders to the Marketing Authorisation Holders (MAH). This represents extra hours for the pharmacist, additional pollution, and traffic for the community. What is sure is that the 300 medications on shortage declared by FAGG/AFMPS are only the top of the iceberg. The reality of shortages is much bigger.
Which are the challenges for 2023?
The problems we are experiencing today are not going to suddenly disappear tomorrow, but there are ways for parallel trade to help with these issues, especially if a more efficient regulatory framework is created. Parallel importers (PI) are a valuable partner in combatting shortages and should be called quicker by the authorities when a shortage is detected. We have years of experience detecting surplus on other European markets and we have the logistics to bring it to Belgium in a timely and efficient manner. To respond quickly to shortages, PI needs to be able to work in a nimble way. A simplified and effective administrative procedure, with no inequalities vis-à-vis regular pharma is needed to ensure that pharmacists and patients get the medicine they need in time.
Concerning the Royal Decree 19/04/2001 update, a revision of the legal framework is required. Compared to other European countries and the framework applicable to regular pharma, we can make the application procedure in Belgium quicker and easier, without impacting patient safety. And by combining with a strict compliance to the procedure by the FAGG. This would reduce the workload for both the parallel import and the FAGG.
Other hurdles to a quick and responsive PI can be found at the level of RIZIV and FOD Economy: if PI and PD wants to be responsive to shortages, parallel importers need to have access to a quicker approval for price and reimbursement. Since the imported products are identical or found to be sufficiently identical by the FAGG to the products that have already received a price and reimbursement on the Belgian market, price and reimbursement should be granted in a quasi-automatic manner.
Special attention should also be given to the Market Entry Agreements (MEA): these are increasingly prevalent in the current pharma landscape. At present, regular pharma have a monopoly of 3 years on products covered by a MEA. Since parallel distributors import the same product from the same originator, there is no reason not to allow parallel distribution products with the same price and reimbursement conditions. Freedom of trade and competition will only benefit to patients and the social security system”.
To know more about our campaign #FillTheGap, follow us on Twitter !